Proteogenix는 Primary Antibody Biosimilar - Research Grade
제품을 전문적으로 생산하는 20년의 경험이 있는 High Quality 제조사입니다.
제품 설명
제품 번호
PX-TA1332
제품 특징
| Product name | Anifrolumab Biosimilar - Anti-IFNAR1 mAb - Research Grade |
|---|---|
| Source | CAS 1326232-46-5 |
| Species | Homo sapiens |
| Expression system | Mammalian cells |
| Purity | >85% |
| Buffer | PBS buffer PH7.5 |
| Delivery condition | Blue ice (+4°C) |
| Delivery Time | 3-5 days if in stock; 3-5 weeks if production needed |
| Storage condition | store at -80°C |
| Brand | ProteoGenix |
| Aliases /Synonyms | Anifrolumab,MDX-1333,MEDI-546,IFNAR1,anti-IFNAR1 |
| Reference | PX-TA1332 |
| Note | For research use only. Not suitable for clinical or therapeutic use. |
| Isotype | IgG1-kappa |
| Clonality | Monoclonal Antibody |
Anifrolumab has been studied to treat Scleroderma. Anifrolumab, or MEDI546, is a human monoclonal antibody of IgG1 type. It is able to bind to interferon (IFN) alpha receptors 1 (IFNAR1). This bond blocks type I IFN signaling. Anifrolumab is carrying three mutations (L234F/L235E/P331S) in the heavy chain. Those mutations aim to reduce the cell surface Fc gamma receptor and potential Fc mediated effector function. Indeed, antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) are mechanisms depending on these parts. Anifrolumab is developed to treat systemic lupus erythematosus (SLE) and lupus nephritis.
SLE is an autoimmune disease which is characterized by its chronicity and mustisystem affections. SLE is evolving by succession of inflammatory phases and remissions. Many evidences are showing the role of type I IFNs in the pathogenesis of this immunologic disease. Increased type I IFN expression and type I IFN-induced cell signalling correlate with SLE severity. Numerous genetic polymorphisms increase susceptibility to SLE by increasing type I IFN signaling.
Type I IFNs family includes IFN-α subtypes, IFN-β, IFN-δ, IFN-ε, IFN-κ and IFN-ω. These interferons and their receptors, the IFN alpha receptors 1 (IFNAR1) and 2 (IFNAR2), form a IFNAR complex with specific functions. This complex is leading to tyrosine phosphorylation of signal transducer and activator of transcription 1 (STAT1) and 2 (STAT2). Once phosphorylated, STAT1 and STAT2 translocate with IFN regulatory factor 9 (IRF9) to the nucleus. Then, they drive IFN-stimulated response element (ISRE) activation. ISRE promotes transcription of multiple IFN-stimulated genes: which leads to the production of proinflammatory and immunomodulatory proteins involved in the host innate immune response to viral infection.
QC & Validation Data
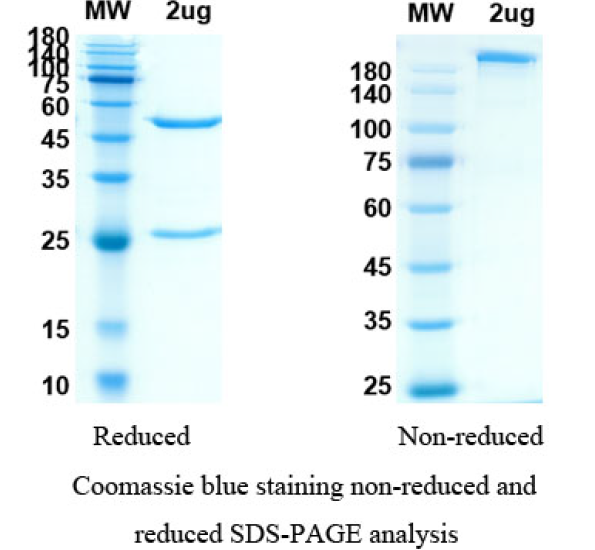
Anifrolumab Biosimilar - Anti-IFNAR1 mAb, on SDS-PAGE under reducing and non-reducing conditions. The gel was stained overnight with Coomassie Blue. The purity of the antibody is greater than 95%.
Publications
ProteoGenix의 모든 제품들을 만나보세요!
Proteogenix - Exclusive Distributor in South Korea "Morebio" 한국 독점 대리점 "모아바이오"